Association
Activities
Overview of KPIA
The association was originally founded in 1948 as the Osaka Pharmaceutical Manufacturers Association (OPMA) and the name of OPMA was changed to the Kansai Pharmaceutical Industries Association (KPIA) on January 1st, 2018.
KPIA is a Regional organization aiming at enhancing common benefits for members and contributing to the realization of “Society of Health and Longevity” by making use of the strengths of Kansai*, and through strengthening joint collaboration, mutual understanding, and enlightenment of stakeholders including members, and through the sound development of the pharmaceutical and related industries.
The KPIA comprises over 300 pharmaceutical marketing authorization holders/ manufacturers and companies engaged in associated businesses, headquartered mainly in the Kansai Area. KPIA is a member of the Federation of Pharmaceutical Manufacturers’ Associations of JAPAN (FPMAJ) as a regional organization.
KPIA conducts its activities through four (4) committees and six (6) study groups and the secretariat, under the direction of the Board of Directors to address problems that operators in the pharmaceutical industries are facing. Specifically, KPIA keeps close contact and cooperation with, and promote mutual understanding with, the Ministry of Health, Labour and Welfare (MHLW), the Osaka Prefectural Government and other Regional authorities in the Kansai Area governing pharmaceutical industries to collect, examine, analyze, and compile information related to pharmaceutical-related laws and regulations and the pharmaceutical industries.
The results of these activities are communicated to members through seminars, newsletters, information network and other events and media to provide information and improve the knowledge of members. KPIA also makes various proposals related to pharmaceutical industries to the national and Regional governments and other public organizations.
Membership Benefits

Use of PRAISE-NET*
*Pharmaceutical Regulatory Affairs Information Service
Participation in Lectures/Seminars organized by KPIA

Participation in Committees/Study Groups

Viewing/Downloading of KPIA’s Monthly Journal on the Web

Purchase of books published by KPIA
Percentage of Members
KPIA’s Membership: 328(as of December 1, 2025)
Business type of KPIA members
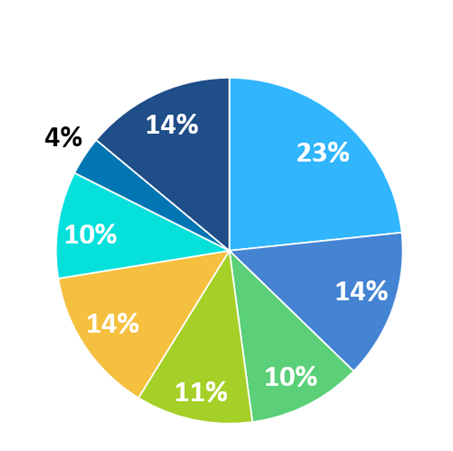
- Innovative pharma
- Generic pharma
- OTC
- API/Excipient/ Reagent
- CMO/CDMO
- CRO
- Diagnostics
- Others
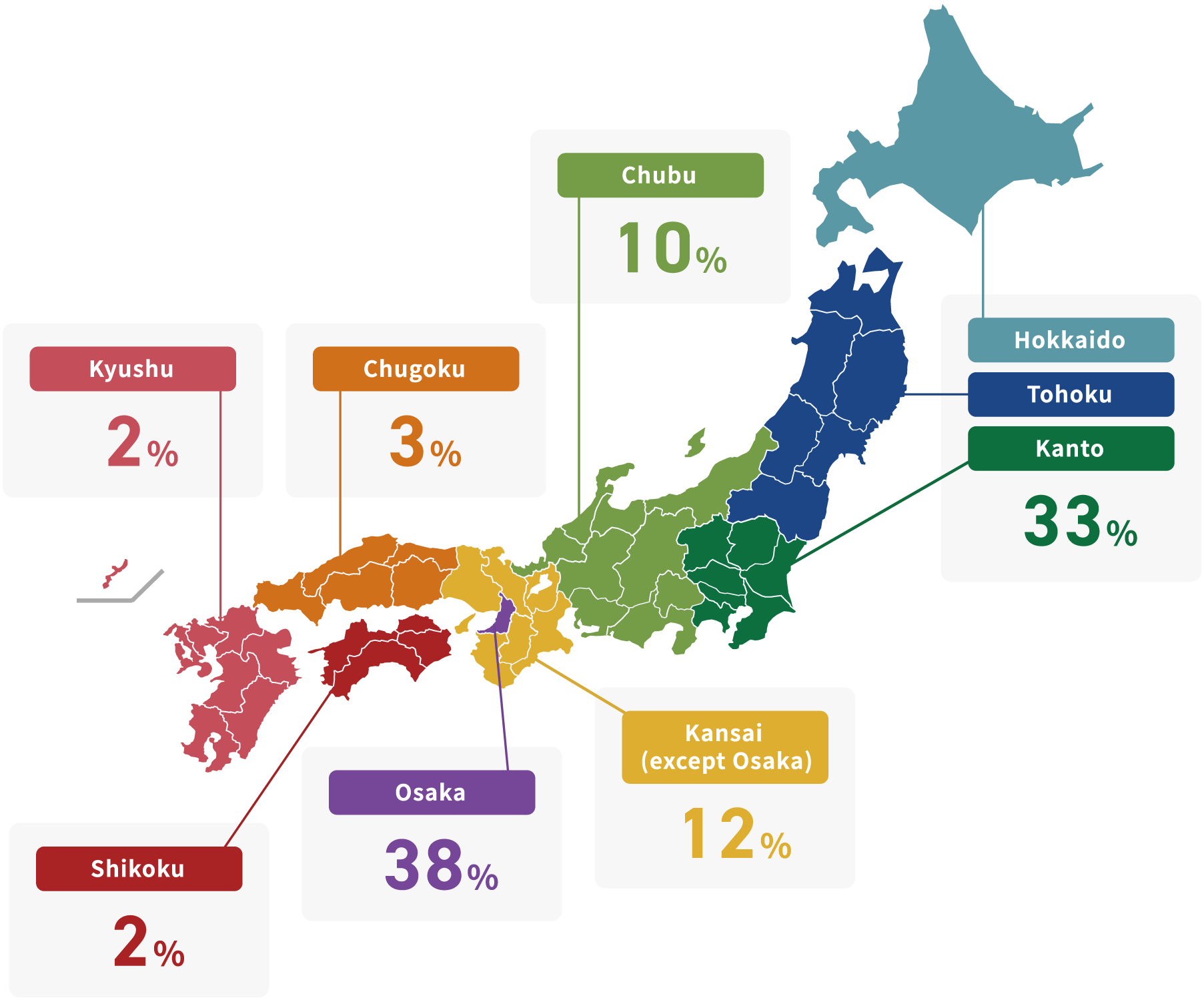
Headquarters location of KPIA members
Activities of Committees and Study Groups
Committees and study groups are formed according to specialized fields and actively work on projects to find solutions for various issues and problems up to which operators in the pharmaceutical industries face. Member companies can join any committees and study groups they are interested in and take part in their activities. Currently, KPIA has the following four (4) committees and six (6) study groups:
1.Pharmaceutical Regulatory Affairs Committee
The Pharmaceutical Regulatory Affairs Committee promotes members’ compliance with the PMD Act. It has formed Sub-Committees, each assigned to a specialized field, including legislation, pharmaceutical regulatory affairs, advertisement/promotion, packaging/labeling, OTC drugs, and pharmaceutical regulations in other countries, to discuss systems related to pharmaceutical laws and regulations, and issues found during the operation by member companies. The Committee also actively participates in projects of the Pharmaceutical Regulatory Affairs Committee of FPMAJ, the upper body of KPIA, to address issues related to the PMD Act.
In FY2025, the Committee focuses on the following activities:
-
Research Study and Proposals Related to Pharmaceutical Laws and Regulations
To solve issues faced by member companies in practicing pharmaceutical businesses, the Committee collects opinions and requests from member companies and conducts research to identify issues in pharmaceutical business practice. Based on these activities, the Committee makes requests to relevant administrative organizations through FPMAJ to get them issue notifications which should clarify measures to be taken.
Furthermore, the committee investigates and studies the trends in the amendment of the PMD Act scheduled for submission to the regular Diet session in 2025, as well as related revisions to government and ministerial ordinances, and provides recommendations to the authorities through the FPMAJ.
It also compiles opinions and requests from member companies regarding pharmaceutical and healthcare regulations and support and incentive measures, and makes proposals through exchanges of opinions with the authorities. - Proactive Proposals Regarding Measures that Help the Development and Growth of Member Companies and Regarding Rationalization of Regulations
The Committee makes proposals for rationalization of regulations and systems to match the globalization of pharmaceuticals and related products. It also conducts research and examinations, and makes proposals regarding modification of regulations to accelerate the launch of next-generation medical treatments such as regenerative medicines and new healthcare products.
- Cooperative Recommendation to MHLW and PMDA
The Committee collects opinions and requests from member companies regarding issues they have found in practicing their operations, such as the approval review of drugs (approval of new drugs, partial changes, and confirmation of change management protocol), inspections (document inspection, on-site inspection, and GMP compliance inspection), consultation services (face-to-face advice, simplified consultation), notification services (a notice of clinical trial plan, minor change notification, and manufacturing notification of pharmaceuticals for export/import), export/import procedures under the PMD Act, and research and development activities. Based on these opinions and requests, the Committee proposes improvements to MHLW and PMDA.
- Activities by Sub-Committees and Working Groups
- Regulatory Affairs Sub-Committee
The Sub-Committee discusses and conducts research on matters (Legal Compliance Guidelines, GDP Guideline, etc.) related to business management (Pharmaceutical Marketing Authorization Holders, Wholesalers), matters related to GMP (Revised Administrative Ordinances on GMP, GMP case studies, etc.) ,and matters related to the application for approval (Considerations of inquiry items about Partial Change Applications, GMP Inspection Applications, Impurity Control, etc.) as themes and topics about the PMD Act and related laws and subjects - Approval and Authorization Sub-Committee
- First Working Group
The Working Group considers implementation of online notifications about a change control of approval document (partial changes and minor changes), and GMP Compliance Inspection, as the subjects highly related to pharmaceutical affairs business. Furthermore, the subcommittee will also examine the potential impact and necessary responses, particularly in areas relevant to the group, depending on the progress of the amendment to the PMD Act scheduled for submission to the 2025 regular Diet session.
The group also organizes seminars to strive for proactive collection of knowledge and information required as a person in charge of pharmaceutical professionals. - Second Working Group
The Working Group selects and conducts research on themes related to the rationalization of entries in the “Specifications and Test Methods” section, applications for GMP compliance inspections of prescription pharmaceuticals, and key considerations for online submissions. - Third Working Group
The Working Group conducts pharmaceutical affairs research on the recommended matters by the MHLW’s expert panel, and contents of CTD Module 1, since these topics are highly related to a pharmaceutical affairs strategy for new active ingredients, as well as to a daily developmental regulatory business. - Working Group for Drug Approval and Licensing Procedures 2026
In preparation for the publication of “Drug Approval and Licensing Procedures”, the Working Group incorporates necessary legislations, notifications, administrative notices to reflect the latest regulatory contents, and review/revise for further improvement to operate their business practice. - Working Group for Approval/Authorization Information
The Working Group makes proposals to improve the usability and function regarding the database of pharmaceutical-related administrative notifications provided by PRAISE-NET. It also maintains and updates the database for person in charge of Approval/Authorization Information currently made available via PRAISE-NET to the Pharmaceutical Regulatory Affairs Committee members, and enhances the database by identifying issues. The working group will also try to increase the efficiency of preparing licensing information summaries using outsourcing and generative AI.
- First Working Group
- Sub-Committee for Advertisement/Promotion
Sub-Committee conducts research on the PMD Act and other related laws and regulations regarding Advertisement/Promotion of pharmaceutical products (ethical and OTC) such as Standards for Fair Advertisement of Pharmaceutical Products, Guidelines for Sales Information Provision Activities for Ethical Drugs, Instruction for preparing the Outline of Product Information Brochure, Guidelines for Adequate Advertisement of OTC drugs, Act against Unjustifiable Premiums and Misleading Representations, and the monitoring program for promotional activities of prescription drugs, focusing on case studies. Sub-Committee holds lecture meetings and other events to actively collect knowledge and information necessary as pharmaceutical professionals. - Sub-Committee for Packaging/Labeling
Sub-Committee conducts research on labeling for ethical and OTC drugs, package design, electronic package inserts, unique identification codes, and interview forms etc. - Sub-Committee for OTC drugs
Sub-Committee conducts research, exchange views and share information about OTC drug inquiries and Q&As on Approval Standard, etc., as topics that are broadly related to daily operations, mainly focusing on issues such as application for approval, marketing and GMP specific to OTC drugs. - Sub-Committee for Overseas Pharmaceutical Regulations
- Group for US and Europe
The Group conducts research mainly on the approval and authorization systems in the US and Europe, and exchanges views and shares information about relevant topics including the systems that promote new drug development in these regions, as well as procedures for facility and product registration in the US, and approval and authorization systems in Europe. - Group for Asia
The Group conducts extensive research on pharmaceutical regulations in Asian countries including China, and exchanges views and shares information on daily operations. Activities are divided into two teams—one focusing on China and the other on countries outside China. Key topics include the appendix on investigational medicinal products in the 2010 revision of the Good Manufacturing Practice (GMP) standards, and Revision 2 of the ASEAN Guidelines on Drug Registration Variations.
- Group for US and Europe
- Regulatory Affairs Sub-Committee
- Holding of Workshops and Seminars
Joint Pharmaceutical Regulatory Affairs Committee between KPIA and PMAT organizes seminars lectured by MHLW and PMDA representatives on the latest movements in pharmaceutical regulations and other topics, and gains answers for questions and requests on the pharmaceutical regulations during the seminars as a help of the pharmaceutical affairs business.
The Committee holds workshops and seminars that are useful for the practical business of the Committee and Sub-Committee members, and are related to the activities of Sub-Committees and Working Groups in a timely manner as appropriate.
2.Technical Research Committee
Technical Research Committee participates in the Japanese Pharmacopoeia Expert Committee (hereinafter “JP Expert Committee”) of PMDA as a sub-committee member and cooperates in preparing the draft of the Japanese Pharmacopoeia and the Guidance for drafting and revising the Japanese Pharmacopoeia. The Committee appropriately conducts surveys on the actual conditions and requests of the member companies to ensure consistency in understanding of the method for quality evaluation, specifications, and test methods for pharmaceutical products between the administrative organizations and the pharmaceutical industry.
The Committee also addresses and solves technical issues related to the Japanese Pharmacopoeia and the marketing approval applications for pharmaceutical products through activities by Working Group organized under the Committee, provision of technical information to the committee member companies, and cooperation/coordination with related committees and organizations.
In FY2025, the Committee Focuses on the Following Activities:
- Cooperation in Revision of the Japanese Pharmacopoeia
・ The Committee sends its associate members to the JP Expert Committee and working groups organized under the JP Expert Committee.
・ The Committee makes requests and suggestions to the administrative agencies and cooperates in the Guidance for drafting and revising the Japanese Pharmacopoeia.
・ The committee cooperates in the preparation of the Guidelines for Drafting Japanese Pharmacopoeia, the Basic Policy for the 20th Revision of the Japanese Pharmacopoeia, as well as the 19th Revision and its First Supplement.
・ The Committee contributes to maintenance and enhancement of the Japanese Pharmacopoeia, improvement of public health, and also to international harmonization and globalization of the Japanese Pharmacopoeia in accordance with the Basic Policy for Preparing the Japanese Pharmacopoeia 19th Edition.
・ The Committee solves technical and practical issues related to the Japanese Pharmacopoeia in cooperation with the related committees and organizations so that the member companies can properly operate and utilize the Japanese Pharmacopoeia. - Cooperation in Revision of Japanese Pharmaceutical Excipients
・ Upon request from MHLW, the Committee sends representatives to the Expert Committee on the Japanese Pharmaceutical Excipients (hereinafter “JPE Expert Committee”) to cooperate in revision of the Japanese Pharmaceutical Excipients. - Provision of Technical Information
・ The Committee reports conclusions and points of discussion from the JP Expert Committee, its working group and the JPE Expert Committee to the member companies in the General committee meeting which is composed of representatives from the member companies. The Committee provides the latest trends in the revision of the Japanese Pharmacopoeia and the Japanese Pharmaceutical Excipients to the member companies and facilitates discissions.
・ The Committee holds lecture meetings on the latest information regarding notification/regulations or analytical technologies with the General committee meeting, providing valuable technical information directly applicable to the practical work of the member companies
・ In cooperation with PMAT, the Committee prepares and publishes the Practical Guide to complement the Guidance for drafting the Japanese Pharmacopoeia, serving as a resource for drafting the Japanese Pharmacopoeia and the developing test methods. - Proposals to the Japanese Pharmacopoeia and Addressing Technical Challenges in Quality Evaluation
・ The Committee, together with the related organizations, considers proposals to adapt the Japanese Pharmacopoeia to the diversification of pharmaceuticals and to improve the efficiency of drafting the Japanese Pharmacopoeia.
・ The Committee seeks solutions to technical issues in the quality evaluation of pharmaceuticals in the Japanese Pharmacopoeia and the marketing approval applications for pharmaceutical products. - Working Group Activity
・ Working Group for Biopharmaceuticals researches quality evaluation methods, specifications and test methods, and other matters of biopharmaceuticals.
・ The achievements are shared with the member companies by reporting the details of its activities in the general committee meeting.
3.Quality Committee
The Quality Committee aims to enhance the Pharmaceutical Quality System by collaborating with the Osaka Prefectural Government and the other Authorities in the Kansai region. It promotes mutual learning through information exchange and mutual enlightenment on GQP and GMP with relevant business organizations and committees within the pharmaceutical industry including the FPMAJ and member companies.
In recent years, the Pharmaceutical Quality System have evolved from an international regulatory harmonization and convergence perspective. Scientific and logical proposals and discussions are essential for ordinances, notifications, and guidelines related to GQP and GMP (including GDP). The Committee actively submits proposals to administrative organizations through FPMAJ.
It also identifies issues related to GQP/GMP and their operation including GDP, and presents requests or proposals to MHLW and PMDA through FPMAJ and the Osaka Prefectural Government. The goal is to address these issues and develop mutual understanding between the administrative organizations and the pharmaceutical industries.
Furthermore, the Committee proactively plans and implements industry-wide measures to regain public trust due to the recent manufacturing and quality violations and repeated recalls.
In FY2025, the Committee focuses on the following activities:
- Identification of Issues and Proposals in Relation to the Inspection/ Guidance of GQP/ GMP and their Operation Including GDP
- Requests and proposals for the revision of PMD Act, as well as efforts to address issues arising after the revision
- Promotion of various measures after the revision of the GMP Ministerial Order
- Revision of PIC/S GMP Guideline and its Annex
- Identification of issues and submission of opinions regarding other administrative notifications related to GQP/GMP/GDP from MHLW or Osaka Prefectural Government, etc.
- Promotion of Cooperation with Authorities Mainly in Kansai Region
- Promotion of Cooperation with Osaka Prefectural Government
- To seek common understanding of issues related to GQP/GMP/GDP through regular information sharing and opinion exchange with Osaka Prefectural Government, and to address challenges.
- Active participation in the “Standard Evaluation Examination Sectional Meeting for Pharmaceutical Products under the Osaka Prefecture Pharmaceutical Affairs Council”, providing the Committee’s opinions on GQP/GMP issues, and disseminating relevant information to member companies.
- Cooperation with the GMP Team under the “Pharmaceutical Affairs Division Manager Meeting in Kansai Region”
– Cooperating with the “GMP Team consisting of the Chiefs of Pharmaceutical Affairs in Kansai Region”, and proactively exchange opinions to enhance the partnerships with Authorities in the Kansai Region.
- Promotion of Cooperation with Osaka Prefectural Government
- Planning and Implementation of Measures as an Industry Organization to Prevent Recurrence of Serious Quality Issues
– With reference to opinions exchanged with the admi
– Th nistrative organizations, the Committee considers short-, medium- and long-term measures, and promotes activities on those measures. - Addressing Practical Issues:
The Committee’s three Sub-Committees (Quality System Research, Operations Case Study, and Education) study and discuss how to address practical issues to reach solutions:
The theme of study at each Sub-Committees in FY2025 are as follows:
Quality System Research Sub-Committee: Digging into the practices of data integrity more deeply
Operations Case Study Sub-Committee: Management of suppliers and outsourcing contractors, including the cooperation between Marketing Authorization Holders and Manufacturers
Education Sub-Committee: Human resource development related to GMP and GQP. - Holding of Lectures, Briefings, and Similar Events
At the biannual general committee meetings, the committee makes reports on recent topics related to its activities, as well as to activities by FPMAJ Quality Committee. It also organizes special lectures by administrative authorities including the Pharmaceutical Affairs Division of Osaka Prefecture. In addition, the committee seeks to promote information exchange and mutual learning through factory tours and similar events. Furthermore, it cooperates with the association’s Training Sessions on Pharmaceutical Affairs and Pharmaceuticals General Marketing Director Study Course.
4.International Business Committee
International Business Committee, in collaboration with KPIA’s secretariat, promotes further globalization of the Japanese pharmaceutical related industries by providing support for the international business activities of member companies.
In FY2025, the Committee focuses on the following activities:
- To promote the collection and sharing of information on overseas Pharmaceutical Related Value Chains, research and development, and regulatory trends.
Among overseas countries, especially in Asian economies (China, India, Korea, Taiwan, ASEAN, etc.), in particular, Japanese companies are gradually expanding their businesses, but they are facing difficulties in making them profitable. Asian countries have been growing substantially as a market, and some Asian countries have become important manufacturers of APIs and other related products. In view of the above, the Committee enhances seminars/lectures, matchmaking/net-working meetings, on-site visits, workshops, information exchange on pharmaceutical related value chains. Regarding Europe and the US, as it is also important for member companies to understand pharmaceutical related R&D and regulatory trends. Furthermore, alongside Asia, Europe and the US, attention is increasingly turning to the Middle East, the Committee continues to collect and share relevant information through seminars. - Exchange with Bio-clusters and Start-ups in Overseas Countries
In cooperation with Osaka Prefectural Government, other regional governments, and related organizations (such as Bio-Community Kansai (BiocK), Foundation for Biomedical Research and Innovation at Kobe, Kyoto Research Park, and Senri Life Science Foundation), the Committee holds business net-working meetings, lectures and other exchanges with overseas biotechnology clusters and start-ups. Additionally, upon request, networking events will be arranged with international delegations visiting Osaka for the Osaka-Kansai Expo. - Promotion of Activities in Cooperation with Stakeholders.
The Committee strengthens cooperation with overseas governmental agencies and trade organizations, Embassies and Consulate Generals in Osaka, MHLW/PMDA, regional governments such as Osaka, Kyoto, and Kobe, JETRO, Chambers of Commerce and Industry in Osaka, Kyoto, and Kobe, Kansai Economic Federation and international committees, FPMAJ, JPMA, JGA, JSMI, and Japanese embassies and Japanese industry groups operating locally. Through holding conferences and networking meetings, the Committee supports the overseas business expansion of pharmaceutical related industries. - Development of Globally Competent Human Resources
To promote overseas business development in pharmaceutical field, it is essential to develop, secure and retain global and foreign human resources. The Committee identifies their needs to support for developing, securing and retaining globally competent human resources, and provides support through relevant seminars and workshops. - Strengthen the Information Distribution to overseas
The Committee strengthens efforts to disseminate information about our activities to overseas stakeholders by reviewing and enhancing the contents of our English website which introduces our activities and additionally, using the Kanyakukyo YouTube channel, newly launched in 2024.
5.Eye Drops Study Group
Eye Drops Study Group conducts research on pharmaceutical regulations and technical issues related to eye drops, and prepares and proposes measures to solve these issues. The Study Group also conducts activities requested by administration or industry organizations, such as research and examination on issues related to eye drops and proposal of solutions, aiming toward improvements of the quality of eye drops and enhancement of the level of industries. The results of its activities are made widely available not only to member companies but also to the public by posting them on KPIA’s website and other methods.
In FY2025, the Study Group focuses on the following activities:
- Activities Regarding Education on Proper Use of Eye Drops
The Study Group continues enlightenment activities related to proper use of eye drops with educational materials created to date such as a “Handbook for Pharmacists”, a “Pamphlet for Patients/General Consumers” and a “Poster for Elementary School Children Regarding Education on Proper Use of Eye Drops”. - Activities to Address Official Documents and Administrative Notifications
The Study Group examines matters requested by FPMAJ and JSMI. Such requests include a review of approval criteria for manufacturing ophthalmic preparations; research on revision of the Japanese Pharmacopoeia General Provisions, the general testing method and reference information; matters related to a prevention of medical accidents; publication of cases in the GMP case book, and research related to administrative notifications. - Research on Issues in Pharmaceutical Regulations and Quality of Eye Drops, and Actions to Address These Issues
The Study Group holds plant tours and various study sessions, as well as conduct questionnaires and exchanges of opinions with the member companies of the Study Group, in order to solve issues related to pharmaceutical regulations, manufacturing control and quality control of eye drops. The Study Group also tries to standardize interpretation and terminology, and consider measures to address these issues, aiming to support learning of knowledge and skills. - Cooperation with Eye Drops Study Group of PMAT
To gather opinions from eye drops industry and facilitate making proposal to the administration and industry organizations, the Study Group cooperates with Eye Drops Study Group of PMAT in conducting joint report meetings on the results of research study and joint opinion exchange meetings. The Study Group holds workshops and study sessions, including tours to production sites of pharmaceutical products and other subjects. It also supports learning of general knowledge and skills related to pharmaceuticals among member companies of KPIA’s and PMAT’s Study Group, and conducts opinion exchanges leading to quality improvement of pharmaceuticals.
6.Intellectual Property Study Group
1) Intellectual Property Study Group establishes each Sub-Committee in the field of patent, patent information and trademark, respectively. Each Sub-Committee selects research topics to collect information and to conduct practical studies. The topic includes various issues of intellectual property systems in terms of patents/trademark examinations/trials related to pharmaceuticals, as well as healthcare that pharmaceutical companies have worked on in recent years. Sub-Committee also addresses issues in the practice of survey and search of intellectual property information. 2) The Study Group also discusses various intellectual property issues with JPO or other relevant agencies, in cooperation with Intellectual Property Study Group of PMAT. The Study Group distributes information and results obtained by these activities to member companies in order to support their intellectual property activities.
In FY2025, the Study Group focuses on the following activities:
- Patent Sub-Committee
The Sub-Committee studies various issues concerning patent systems in Japan and overseas.
The Sub-Committee collects and reviews information in Japan in a timely manner, mainly regarding practical aspects of examination and appeal by JPO as needed, with paying attention to the trends after the revision of the Patent Act or the Examination Guidelines, to provide member companies with results of these activities. Hearing opinions from member companies, the Sub-Committee submits requests and opinions to JPO or other relevant administrative organizations in cooperation with Intellectual Property Study Group of PMAT, as necessary.
The Sub-Committee also collects pharmaceuticals and healthcare related information as well as precedents and theories related to a patent law in each overseas country, and conducts research and analysis on the information. It identifies issues that the pharmaceutical industry in Japan has been facing in recent years, and proposes actions to them. - Patent Information Sub-Committee
The Sub-Committee is suspended due to the lack of committee member companies wishing to participate in it. - Trademark Sub-Committee
The Sub-Committee is suspended due to the lack of committee member companies wishing to participate in it.
7.Medical Information Service Study Group
The Medical Information Service Study Group handles issues occurring in customer service departments of pharmaceutical companies that directly receive inquiries from medical professionals such as medical doctors and pharmacists, patients, and general consumers. The Study Group works to acquire not only product knowledge regarding its own products, but also a wide range of peripheral knowledge, including telephone communication skills and know-how in call center operations, and holds various study sessions and workshops so that one can respond to a wide variety of inquiries.
As part of its social contribution activities, the group is registered as a Sukoyaka Partner with the City of Osaka and actively engages in related initiatives. Since FY2024, the group has been conducting outreach lectures titled “How to Take Your Medicine” at local community centers in Hirano Ward, Osaka City, aimed at promoting the proper use of pharmaceuticals among general consumers. These sessions will continue in FY2025.
In FY2025, the Study Group focuses on the following activities:
- Activities of Study Group of Whole and Individual Sub-Committee
The Whole Study Group works to promote mutual understanding among member companies by sharing with the members the information obtained through attendance of the representatives from the “Liaison Meeting for Medical Information Service Manager” hosted by PMDA and the Sub-Committee Meeting for Medical Information Service under Pharmacovigilance Study Committee of FPMAJ.
The Study Group forms the Information Review Study Working Group and the Case Study Working Group under the Study Group and makes great efforts with mutual development as well as self-improvement through monthly regular meetings of these groups.
Information Review Study Group encourages self-improvement through plan, development, and management of various study groups and workshops. It also strives to gain a reputation as attractive Study Group activities.
Case Study Working Group works to improve communication skills through investigation of consultation cases, and shares their results with member companies so that they can be used as educational materials in each member company. - Holding of Seminars and Training Sessions
The Study Group holds the following events to encourage member companies to acquire and broaden their knowledge and contribute to the improvement of the committee members’ skills.
- Pharmaceutical Consultant Forum
- Inquiry response skill up training
- Site tour - Holding of Study Workshops to Acquire a Wide Range of Knowledge
The Study Group identifies needs of Group members, holds timely study workshops on topics of interest to Group members in order to obtain various knowledge. - Cooperation with Related Organizations
The Study Group considers to collaborate with FPMAJ, PMAT, and JPMA regarding common issues (such as a corresponding to customers).
8.Pharmacovigilance Study Group
Pharmacovigilance Study Group participates in discussion sessions and projects organized by administrative organizations, FPMAJ and other organizations to collect latest information on safety measures for pharmaceutical products. It also supports member companies’ safety activities by providing feedback about information to member companies in a timely and appropriate manner and distributing notifications issued by the administrative organizations. The Study Group exchanges and discusses new issues related to drug safety measures mainly through its standing committee. Based on the results of these activities, it makes proposals to administrative organizations, FPMAJ and other related organizations as necessary. It also plans and conducts workshops and training sessions as part of educational activities. Information Sub-Committee under the Study Group supports member companies to take prompt and appropriate post-marketing safety management by raising awareness of the need to improve the ability of the person in charge of operations to assess side effects of pharmaceutical products.
In FY2025, the Study Group focuses on the following activities:
- Activities Related to New Post-Marketing Safety Management Operating Procedures
The Study Group works to collect information in a timely manner from the administrative organizations and FPMAJ (participation in the Safety Committee of FPMAJ) regarding the revision of PMD Act scheduled for FY2025 and new movements of pharmacovigilance, supports safety measures of member companies regarding the post-marketing safety management operating procedures by giving feedback about information to member companies, and makes proposals to FPMAJ, etc. if necessary. - Activities Related to Compliance (Safety) by Marketing Authorization Holders
The Study Group supports member companies by collecting and providing information related to on-site inspections of GVP conducted by local governments and compliance inspections of GPSP by PMDA, and other related information in a timely manner so that they can smoothly comply with regulatory requirements. - Activities Related to the Drug Information Service System
The Study Group participates in FPMAJ information service system project, and provides opinions on PMDA’s “Pharmaceuticals and Medical Devices Information Providing Website”. The Study Group gives feedback about the information to member companies to ensure that they learn necessary matters. - Activities Related to Regional Pharmaceutical Administration
The Study Group participates in the Subcommittee for Evaluation of Standards for Pharmaceuticals and Related Products and the Subcommittee for Measures to Ensure Proper Sales of Pharmaceuticals under the Osaka Prefecture Pharmaceutical Affairs Council, provide recommendations, and gives feedback of information to member companies to keep them informed about it. - Enlightenment on Drug Safety Measures
The Study Group enhances the awareness of importance of drug safety measures through planning and holding seminars on drug safety by external lecturers as well as lecturing at the Pharmaceutical Regulatory Affairs Workshops and the Pharmaceuticals General Marketing Director Study Course.
Information Sub-Committee, regularly scheduled monthly meetings, supports member companies to take proper actions without omissions by improving the abilities of the person in charge of operations to evaluate safety management information and preparing appropriate reporting forms to the administrative organizations through reviews of serious adverse reaction cases, research reports, and action reports by group work. By using published pharmaceutical product RMPs, the Sub-Committee also examines the components of these RMPs and the evidence of safety specification items and provides support to member companies to deepen their understanding of RMPs. The Study Group works to improve the response capability of member companies through providing topics related to pharmacovigilance from standing members and sharing of understanding of issues in safety management operations and related notifications. The Sub-Committee also seek to strengthen the GVP and GPSP systems of Sub-Committee member companies.
In addition, the materials reviewed in the Information Subcommittee—such as serious adverse event cases, research reports, action reports, and RMP analyses—are published as deliverables and shared with Sub-committee member companies.
9.MR Education and Training Study Group
Medical Representatives (MR) Education and Training Study Group supports member companies in conducting effective MR education and training to enhance and strengthen the contents of education and training programs of member companies, aiming to improve the quality of introductory education and continuing education of MR.
And the Study Group also strives to reflect requests of member companies at the various committees in the MR Education & Accreditation Center of Japan, to the preparation of forthcoming MR accreditation system revision in FY2026,
In FY2025, the Study Group focuses on the following activities:
- Pilot Implementation of the Accreditation Standards for Practical Training
The proposed revision to the MR Certification System, scheduled to take effect in FY2026, introduces a lifelong education framework referred to as a “two-tiered” system, separating foundational education from practical training.
・Foundational Education:
This is self-directed learning aimed at acquiring and maintaining basic knowledge (the first tier). Subjects include pharmaceutical information, diseases and treatments, and general MR topics. Learners take a CBT (Computer-Based Testing) exam and receive certification upon passing. Additionally, individuals complete designated self-study programs provided by the MR Certification Center.
・Practical Education:
This is company-led training (the second tier), with subjects including ethics, safety management, skills, and other topics deemed necessary by the company. Practical education consists of introductory and continuing training. Introductory training involves educational sessions, performance assessments, and completion reports, culminating in certification. Continuing education refers to ongoing training conducted by the company.
A new set of certification standards for practical education will be established, and On-the-Job Training (OJT) based on these standards will be officially implemented starting in FY2026. In preparation, a pilot program is conducted during FY2025, with efforts made to share information regarding the operation of the practical education certification standards, including training programs and methods for evaluating outcomes. - MR Educator Course
MR educator course sets up a curriculum related to MR accreditation system and MR textbooks to support an education and training program of the member companies. To provide the most up-to-date information, lecturers are selected from the study group member companies who are familiar with the contents of training, and for some subjects, outside lecturers are selected. In addition, the Study Group makes efforts to publicize this course by announcements and posting posters on KPIA’s website and in KPIA’s bulletin. - Activities related to MR Accreditation Center of Japan
In collaboration with MR Accreditation Center of Japan, the following activities are carried out:
– To send a representative from the Study Group to Education and Training Committee of MR Accreditation Center of Japan and to attend regularly scheduled meetings of Education and Training Committee to collect appropriate information.
– To collect the latest information at MR forums, education and training promotors’ meetings, education and training system accreditation workshops and reflect it in an education and training program of the member companies.
10.Clinical Trial Promotion Study Group
Clinical Trial Promotion Study Group collects issues related to clinical trials from member companies and holds active opinion exchange meetings to find concrete solutions for these issues that match the actual conditions of clinical trials. The members can bring back the outcomes of these activities to their companies.
In addition, the outcomes obtained through these opinion exchange meetings that can be shared among member companies are also compiled into publications.
In FY2025, the Study Group focuses on the following activities:
- Promotion of Working Group Activity
Clinical Trial Sub-Committee, the sub-sector of the Study Group, has formed working groups that examine various subjects related to clinical trials. In FY2025, continuing from FY2024, The Sub-Committee sets up working groups on each of the following four (4) themes. The group members share the latest information, knowledge and experience related to GCP with each other and strive for mutual improvement. Through these activities of the working groups, it supports effective and efficient clinical trial activities of member companies.
Theme 1: Guidance for Preparation of Quality Management Plans and Reports for Clinical Trials
Theme 2: Considerations for Future GCP Renovation
Theme 3: Expanding the Possibilities of Medical Affairs
Theme 4: Bioequivalence Studies of Generic Drugs (Generics) in Response to ICH M13A Guideline “Bioequivalence for Immediate-Release Solid Oral Dosage Forms” - Support Activities for the Association Business
As in the past, the Study Group provides lecturers on GCP-related themes at the pharmaceutical regulatory affairs workshops held by KPIA according to the annual curriculum in order to contribute to improving the knowledge of the person in charge of operations in member companies. - Cooperation with Related Parties
The Study Group cooperates with MHLW, PMDA, Osaka Prefectural Government, Clinical Trial Network Osaka, Japan Society of Quality Assurance, Osaka Medical Association, and other related parties, and disseminates useful information obtained from relevant organizations.
It also promotes cooperation with the organizations, including publicity of their events at their requests.
Abbreviation
– BiocK: Biocommunity Kansai
– FPMAJ: Federation of Pharmaceutical Manufacturers’ Association
– GCP: Good Clinical Practice
– GDP: Good Distribution Practice
– GMP: Good Manufacturing Practice
– GQP: Good Quality Practice
– IT: Information Technology
– JETRO: Japan External Trade Organization
– JGA: Japan Generic Medicines Association
– JMACCT: Center for Clinical Trials, Japan Medical Association
– JPMA: Japan Pharmaceutical Manufacturers Association
– JPO: Japan Patent Office
– JSMI: Japan Self-Medication Industry
– KPIA: Kansai Pharmaceutical Industries Association
– MHLW: Ministry of Health, Labour and Welfare
– MR: Medical Representative
– MRA: Japan-EU Mutual Recognition Agreement
– OTC drug: Over-the-Counter drug
– PACMP: Post-Approval Change Management Protocol
– PIC/S: Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme
– PMAT: Pharmaceutical Manufacturers’ Association of Tokyo
– PMD Act: Act on Securing Quality, Efficacy and Safety of Pharmaceutical Products and Medical Devices (Pharmaceuticals and Medical Devices Act)
– PMDA: Pharmaceuticals and Medical Devices Agency
– PMRJ: Pharmaceutical and Medical Device Regulatory Science Society of Japan
– PRAISE-NET: Pharmaceutical Regulatory Affairs Information Service-Network
– R&D: Research and Development
– RMP: Risk Management Plan
- HOME
- Association Activities



